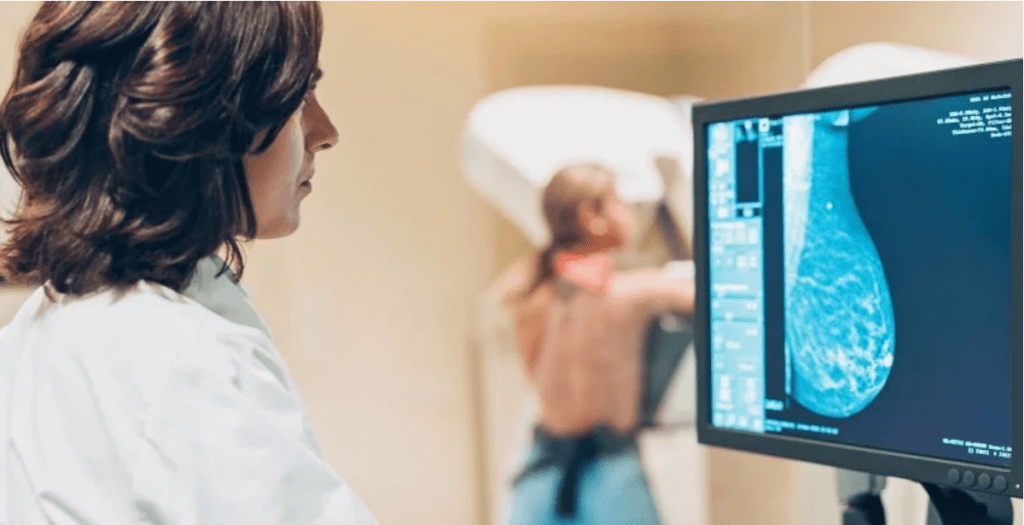
Background
A sizeable proportion of premenopausal breast cancer cases are attributable to having dense breasts. Studies have shown that a decrease in mammographic breast density translates to a reduction in breast cancer incidence. Hence, interventions to reduce mammographic breast density could prevent breast cancer. However, adult dietary and lifestyle modifications have not been shown to reduce mammographic breast density. Therefore, identifying a pathway that can be targeted to reduce breast density and breast cancer incidence is crucial. The receptor activator of nuclear factor-κB (RANK) pathway is important in breast development and progesterone signaling. The RANK pathway also activates downstream signaling cascades involved in breast cancer and has been shown to be positively associated with mammographic density. A well-tolerated RANKL antibody (denosumab) is already in clinical use to prevent fractures in postmenopausal women.
Study Goal
Our goal in this Phase II clinical trial is to perform a randomized clinical trial to quantify the effect of RANKL inhibition on mammographic breast density in 210 premenopausal women with dense breasts. Study findings could open up additional therapeutic approaches in primary breast cancer prevention for premenopausal women with dense breasts, who do not have a dominant genetic predisposition.
Eligibility
You may be eligible to participate if you:
- Are premenopausal
- Aged 40 years and older
- Have dense breasts
- Never had cancer
What will I be asked to do?
If you agree to participate, you will:
- Continue your routine mammograms at baseline, 12 months, and 24 months (allowing the study team access to your mammogram images)
- Complete a urinary pregnancy test at baseline and 6 months, prior to intervention
- Complete a survey that should take no longer than 30 minutes
- Have your blood drawn at the first study visit, 6 months, and at 12 months
- Receive a breast biopsy performed by a breast radiologist at the first study visit and at 12 months
- Be randomized to receive an injection (denosumab or placebo) underneath your skin in the upper arm at the first study visit and at 6 months
- Take Calcium and Vitamin D supplements provided by the research team for 12 months
Participant Updates

Since beginning our study in 2019, we have enrolled 162 women. Of these women, 105 have completed their 12-month visit, meaning they have completed two breast biopsies and injections. We thank all the women who have enrolled in our study and invite any women who are interested to learn more about our previous participants’ experiences. Click Here for Participant Experiences
Coordinator contact information:
“Emmy” Suleepon Uttamapinan
Phone: (314) 747- 9992
Email: ranklstudy@wustl.edu

TRIDENT Trial Recruitment (May 2024)
| RACE | NUMBER (%) |
| Non-Hispanic White | 158 (81%) |
| Non-Hispanic Black | 27 (14%) |
| Asian | 4 (2%) |
| Hispanic | 4 (2%) |
| Other | 2 (1%) |
| TOTAL | 195 (100%) |
Funding: NIH/NCI R37CA235602 (PI: Toriola)
More information on the TRIDENT Trial is available on Clinical Trials.gov here
The TRIDENT Clinical Trial was recently featured in the news! Click here to see the article.